STAGE 2_2023
In the second stage of this project we aimed to evaluate the impact of ELK3 transcription factor in 3 interconnected metastasis-related cellular processes: cellular migration, the epithelial-to-mesenchymal transition (EMT) and in acquiring a cancer stem cell (CSC) phenotype, by using the genetically modified cell lines obtained during the first phase of the project.
The migration capacity of all cell lines was evaluated in a microfludic approach, in PDMS microfluidic devices fabricated using the soft lithography technique. These devices consisted in a central well connected with the media around the device through two arrays of 50 parallel channels, each channel being 600 µm long and 10 µm wide (Fig. 4A). The microfludic devices were coated with type IV collagen, to mimic some of the extracellular matrix components. The cell behavior was monitored for 24h and imaged at 10 min intervals with a Nikon microscope equipped with an incubation chamber. The migration capacity was monitored based on 2 parameters: migration speed (average displacement over 10 min time intervals), and migration persistence (net displacement over total distance travelled). Our results show that ELK3 overexpression stimulates the migration capacity of triple-negative breast cancer cell lines, increasing both migration speed (Fig. 4B) and persistence (Fig. 4C).
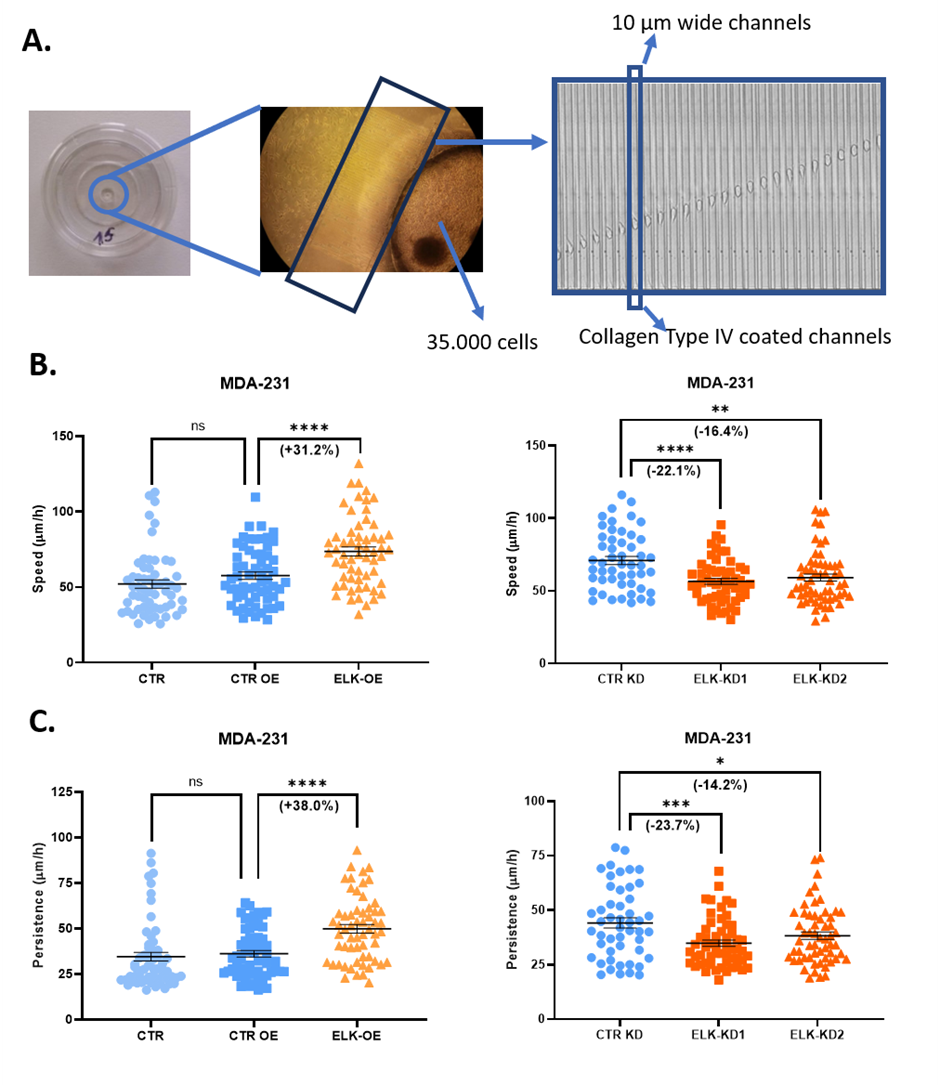 Fig. 1 The cell migration capacity in 3D microfluidic devices (A), in terms of speed (B) and persistence (C), for MDA-231 cells overexpressing ELK3 (ELK-OE) or with inhibited ELK3 expression (ELK-KD1 and ELK-KD2).
Fig. 1 The cell migration capacity in 3D microfluidic devices (A), in terms of speed (B) and persistence (C), for MDA-231 cells overexpressing ELK3 (ELK-OE) or with inhibited ELK3 expression (ELK-KD1 and ELK-KD2).
The phenotype on the epithelial-mesenchymal continuum was assessed by flow-cytometry, both before and after ELK3 overexpression and knockdown. In this assay, specific markers for either the epithelial or mesenchymal phenotypes, mainly E-cadherin, N-cadherin, and pancytokeratin, were used in order to differentiate the two phenotypes and the cell subpopulations. These experiments were performed S3e Cell Sorter (BioRad). Epithelial markers were found increased in cells characterized by ELK3 knockdown (E-cadherin in MDA468_ELK_KD1 and MDA468_ELK_KD1; Pancytokeratin in MDA231_ELK_KD1 and MDA231_ELK_KD2), suggesting the ELK3 involvement in triggering EMT in triple-negative breast cancer cells.
The stemness potential of all cell lines, both parental and transformed, was assessed by a mammosphere formation assay. This assay is widely used for its ability to retrospectively identify sphere-forming cells that develop from single stem cell-like clones, allowing the estimation of the sphere forming efficiency (SFE) 38. After cultivating a small number of cells in a mammosphere induction media for 14 days, the stemness potential of each cell line was evaluated based on the average diameter and number of spheroids formed (cell conglomerates with diameters grater then 50 µm or 60 µm). Our results showed that ELK3 overexpression increases the stem capacity of MDA-231 cells, the ELK-OE cell line forming significantly more and larger spheroids then control cells (Fig. 2)
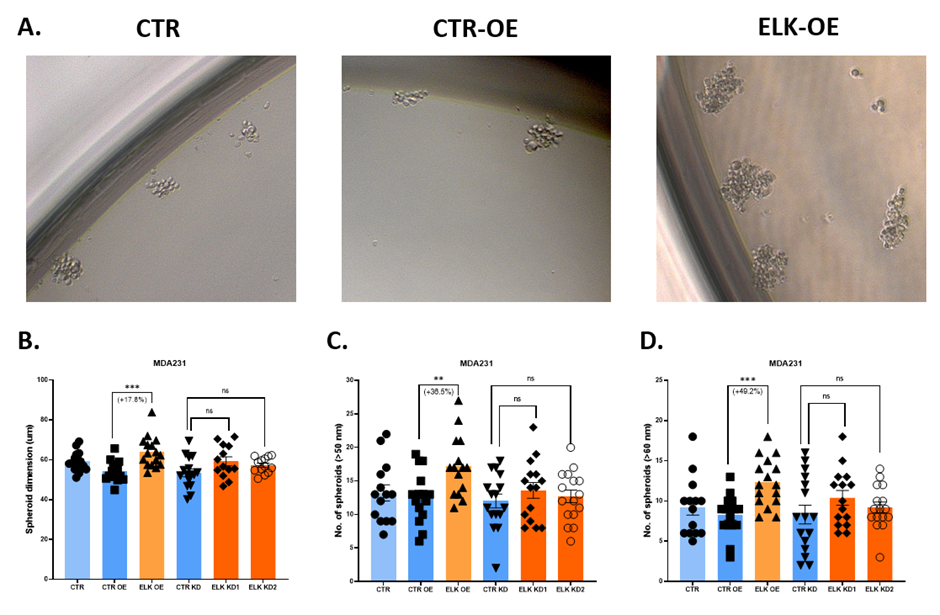 Fig. 2 The stemness potential of MDA-231 cells overexpressing ELK3 (ELK-OE) or with inhibited ELK3 expression (ELK-KD1 and ELK-KD2) in mammosphere formation assays (A), based on the dimensions (B) and number (C and D) of spheroids formed.
Fig. 2 The stemness potential of MDA-231 cells overexpressing ELK3 (ELK-OE) or with inhibited ELK3 expression (ELK-KD1 and ELK-KD2) in mammosphere formation assays (A), based on the dimensions (B) and number (C and D) of spheroids formed.
The migration capacity of all cell lines was evaluated in a microfludic approach, in PDMS microfluidic devices fabricated using the soft lithography technique. These devices consisted in a central well connected with the media around the device through two arrays of 50 parallel channels, each channel being 600 µm long and 10 µm wide (Fig. 4A). The microfludic devices were coated with type IV collagen, to mimic some of the extracellular matrix components. The cell behavior was monitored for 24h and imaged at 10 min intervals with a Nikon microscope equipped with an incubation chamber. The migration capacity was monitored based on 2 parameters: migration speed (average displacement over 10 min time intervals), and migration persistence (net displacement over total distance travelled). Our results show that ELK3 overexpression stimulates the migration capacity of triple-negative breast cancer cell lines, increasing both migration speed (Fig. 4B) and persistence (Fig. 4C).
 Fig. 1 The cell migration capacity in 3D microfluidic devices (A), in terms of speed (B) and persistence (C), for MDA-231 cells overexpressing ELK3 (ELK-OE) or with inhibited ELK3 expression (ELK-KD1 and ELK-KD2).
Fig. 1 The cell migration capacity in 3D microfluidic devices (A), in terms of speed (B) and persistence (C), for MDA-231 cells overexpressing ELK3 (ELK-OE) or with inhibited ELK3 expression (ELK-KD1 and ELK-KD2).The phenotype on the epithelial-mesenchymal continuum was assessed by flow-cytometry, both before and after ELK3 overexpression and knockdown. In this assay, specific markers for either the epithelial or mesenchymal phenotypes, mainly E-cadherin, N-cadherin, and pancytokeratin, were used in order to differentiate the two phenotypes and the cell subpopulations. These experiments were performed S3e Cell Sorter (BioRad). Epithelial markers were found increased in cells characterized by ELK3 knockdown (E-cadherin in MDA468_ELK_KD1 and MDA468_ELK_KD1; Pancytokeratin in MDA231_ELK_KD1 and MDA231_ELK_KD2), suggesting the ELK3 involvement in triggering EMT in triple-negative breast cancer cells.
The stemness potential of all cell lines, both parental and transformed, was assessed by a mammosphere formation assay. This assay is widely used for its ability to retrospectively identify sphere-forming cells that develop from single stem cell-like clones, allowing the estimation of the sphere forming efficiency (SFE) 38. After cultivating a small number of cells in a mammosphere induction media for 14 days, the stemness potential of each cell line was evaluated based on the average diameter and number of spheroids formed (cell conglomerates with diameters grater then 50 µm or 60 µm). Our results showed that ELK3 overexpression increases the stem capacity of MDA-231 cells, the ELK-OE cell line forming significantly more and larger spheroids then control cells (Fig. 2)
 Fig. 2 The stemness potential of MDA-231 cells overexpressing ELK3 (ELK-OE) or with inhibited ELK3 expression (ELK-KD1 and ELK-KD2) in mammosphere formation assays (A), based on the dimensions (B) and number (C and D) of spheroids formed.
Fig. 2 The stemness potential of MDA-231 cells overexpressing ELK3 (ELK-OE) or with inhibited ELK3 expression (ELK-KD1 and ELK-KD2) in mammosphere formation assays (A), based on the dimensions (B) and number (C and D) of spheroids formed. 